RESEARCH TOPICS
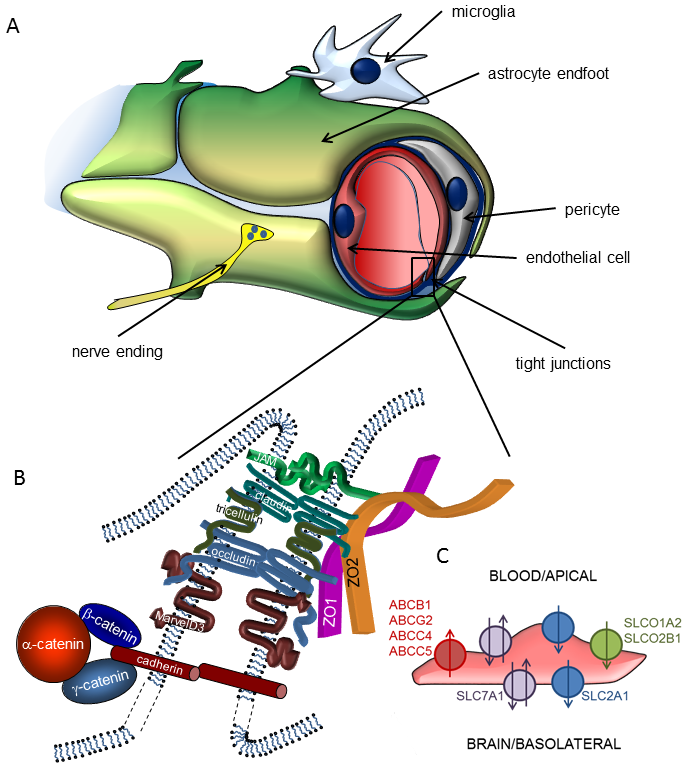
Energy supply of the central nervous system (CNS) is provided by a very dense vascular system with an average distance of 40-50 µm between neighbouring capillaries in the human brain. This implies that almost all neurons are in the close vicinity of a capillary, so that the concept of neurovascular unit (NVU) was coined, emphasizing the inseparable character of neural and vascular functions. Accordingly, the NVU represents a close structural and functional relationship (coordinated action) of microvascular endothelial cells, pericytes, glial cells and neurons.
Main functions of the NVU are formation of the blood-brain barrier (BBB) and neurovascular coupling. The BBB is a highly selective permeability barrier that separates the circulating blood from the extracellular fluid in the CNS, while neurovascular coupling refers to the time- and space-restricted changes in cerebral blood flow in response to neuronal activity.
By strictly regulating the molecular and cellular traffic between the blood and the brain, the BBB substantially contributes to the homeostasis of the CNS. The barrier itself is formed by endothelial cells of the cerebral microvasculature, which acquire special barrier characteristics in the brain microenvironment. Continuous tight junctions (TJs) interconnecting cerebral microvascular endothelial cells seal the paracellular cleft, forcing most molecular and even cellular traffic to use the transcellular, highly regulated way of transport or transmigration. In addition, restricted endocytosis, intracellular enzymes and efflux transporters of the ATP-binding cassette family contribute to the barrier. By sharing important regulatory functions, pericytes and astrocytes are also integral parts of the BBB.
-

Pattern recognition receptors and inflammasomes of cells of the NVU. Role of the NVU in neuroinflammation
-
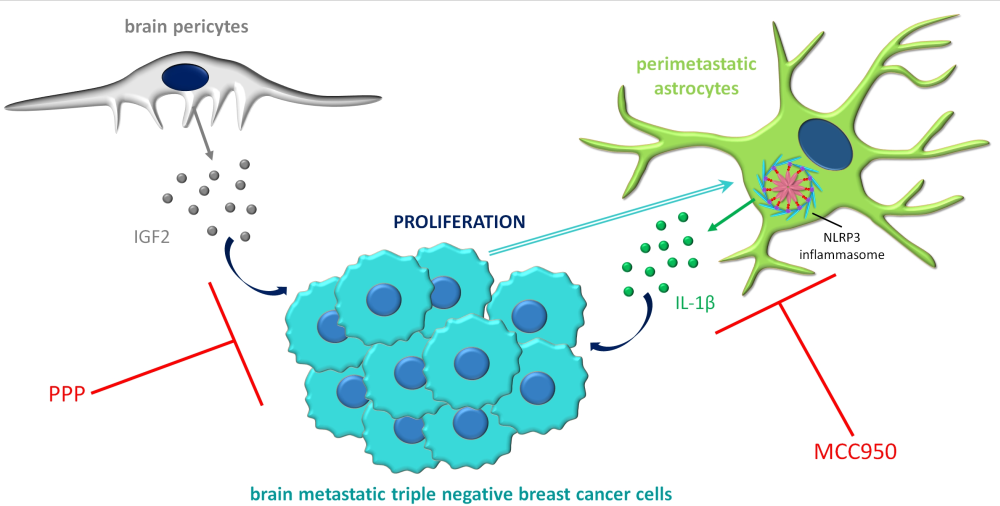
Cross-talk of tumour cells with elements of the NVU during brain metastasis formation
-
Ageing and regeneration of the NVU
-

The NVU in health and disease
Neuroinflammation is a common feature of all brain pathologies, including neurodegenerative diseases, stroke, infections and trauma, and also ageing. Recent data indicates that besides cells of the immune system, resident cells of the CNS actively participate in the induction and propagation of neuroinflammation. By expressing pattern recognition receptors (PRRs) – Toll-like and NOD-like receptors (TLRs and NLRs) –, these cells can sense pathogen- and damage-associated molecular patterns (PAMPs and DAMPs), can activate inflammasomes and release inflammatory cytokines or other pro- and anti-inflammatory agents. All elements of the neurovascular unit (NVU), including glial cells, neurons and also pericytes and microvascular endothelial cells of the blood-brain barrier (BBB) are involved in the inflammatory reaction. Nevertheless, BBB dysfunction is a common feature of neurological disorders, having a key impact on immune cell entry into the CNS.
Our project is focusing on the role of the cells of the NVU in sensing pathogens and tissue injury, and responding to these by activation of TLRs, NLRs and inflammasomes. Our mission is to unravel mechanisms related to the neuro-immuno-vascular axis which may form the basis of future therapeutic approaches.
Despite the potential obstacle represented by the BBB for extravasating malignant cells, metastases – originating primarily from lung cancer, breast cancer and melanoma – are more frequent than primary tumours in the CNS. The brain environment may be very hostile to metastatic cells; however, tumour cells which can adapt to the specific requirements can exploit several advantages, including dense vascularization, supporting factors and shielding against the immune system and drugs.
In the framework of the project, we are studying mechanisms related to endothelial cells, pericytes and glial cells during two specific steps of brain metastasis formation, transmigration of the tumour cells through cerebral capillaries and survival in the brain environment. We are dedicated to understanding the complex cross-talk between tumour cells and host cells in the brain, which is essential for the identification of new therapeutic targets in this devastating disease.
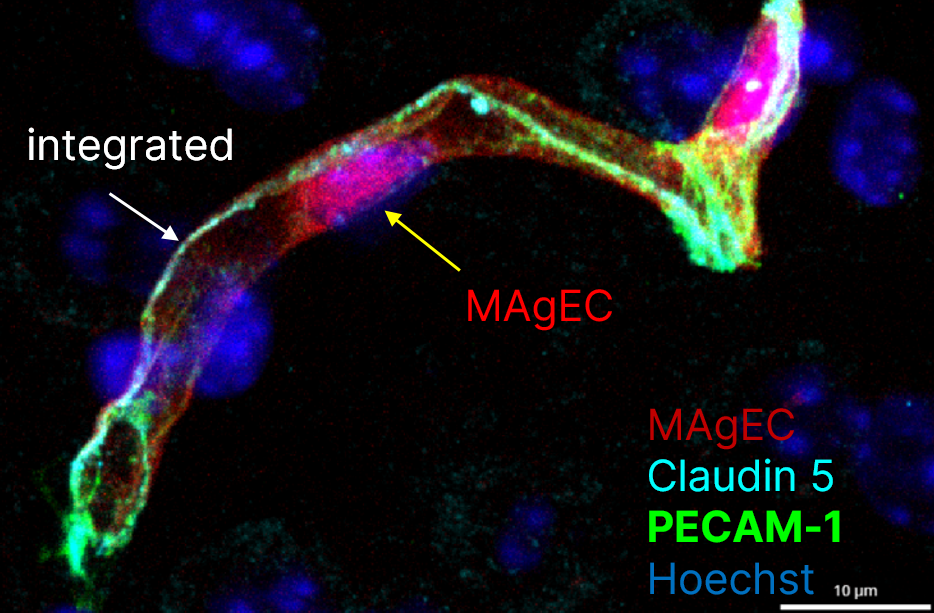
Maintenance of a proper microcirculation in the brain is prerequisite of a healthy brain. Ageing is characterized by morphological and functional changes of the brain microvasculature, which play critical roles in age-related CNS pathologies, including stroke and neurodegenerative disorders. On the one hand, local reduction of microvascular blood flow may have severe consequences on the glucose and oxygen supply necessary for neural function. On the other hand, increased permeability of the BBB leads to accumulation of toxic substances and induction of a low-grade inflammation in the brain, which stays at the basis of all age-related diseases. The decline in neurological function observed in ageing is exacerbated or possible even based on an increase in the number of senescent endothelial cells. Senescent cells exhibit functional changes, e.g. are incapable of proliferation and thus incapable of repairing damaged endothelium or form new vessels and develop senescence-associated secretory phenotype secreting large amounts of molecules that accelerate tissue ageing. However, circulating precursor cells are able to incorporate into the vessel wall and replace damaged cells.
Here, we aim to understand the role of the cells of the NVU, especially of endothelial cells and pericytes, in age-related structural and functional alterations of the cerebral microcirculation. We also aim at assessing the benefits of cellular renewal on the functionality of the NVU.
The NVU is involved in almost all CNS diseases. On the one hand, pathologies of the brain frequently imply impairment of the BBB and of neurovascular coupling. On the other hand, the BBB represents a substantial obstacle to the entry of therapeutic agents into the brain.
We are generally interested in understanding changes in barrier functions of cerebral endothelial cells (including tight junctions and transporters) in pathological conditions, regulation of cerebral blood flow, signalling pathways activated in cells of the NVU, drug delivery to the brain and heterogeneity of the BBB.
