INFRASTRUCTURE AND METHODS
- in vitro and in vivo models, transgenic animals
- isolation and culture of cells of the NVU (cerebral endothelial cells, pericytes and astrocytes) from different species (rat, mouse, porcine, human); several types of tumour cells (human and mouse melanoma and breast cancer cells); mono- and co-culture models
- biochemistry and molecular biology methods (real-time PCR, Western-blot, immunofluorescence)
- impedance measurement systems:
- cellZscope (nanoAnalytics, Münster, Germany)
- xCELLigence® RTCA instrument (ACEA Biosciences, San Diego, CA, USA)
- advanced microscopy methods:
- two-photon microscopy
- super-resolution (STED) microscopy
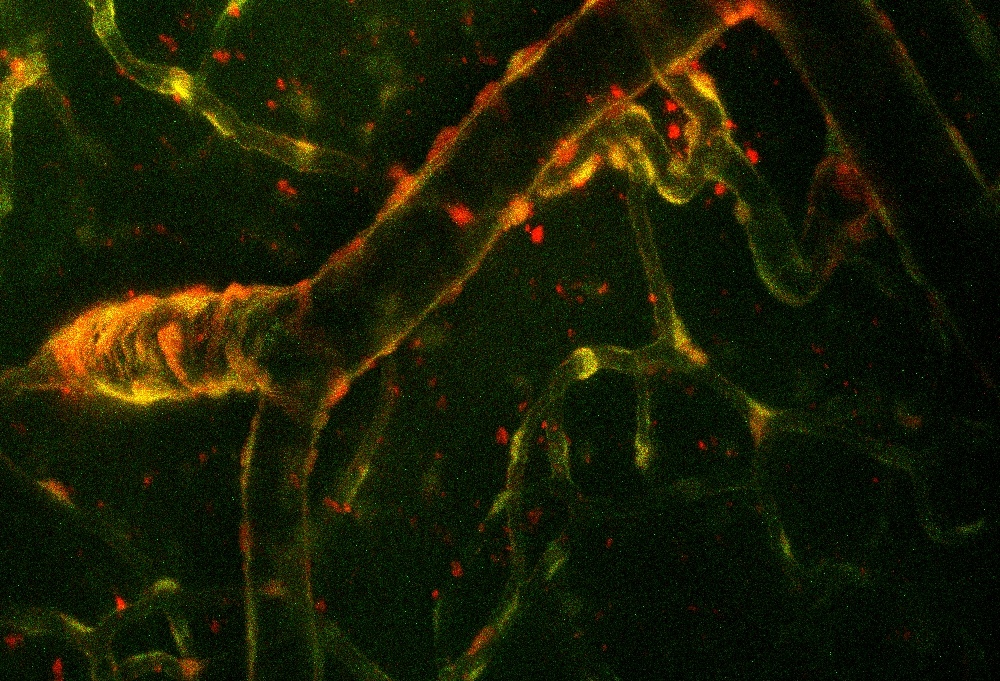
Two-photon microscopy
This fluorescence imaging technique applies near-infrared excitation and multi-photon absorption, allowing for deep tissue penetration with minimized scattering and photo-toxicity. We are using our microscope to image vascular and other cells in the brain tissue of living animals. Our Femto2D Dual microscope (Femtonics, Budapest, Hungary) coupled with a Mai Tai HP Ti:sapphire laser (Spectra-Physics, San Jose, CA, USA) is equipped with a galvanometric and a resonant scanner for high accuracy and high speed imaging of living tissues.
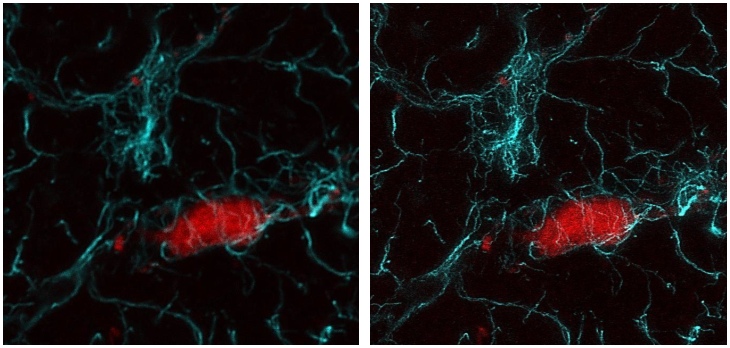
Stimulated emission depletion (STED) microscopy
This technique overcomes the diffraction limit by de-exciting the fluorophores outside the central area of the focal spot using a red-shifted doughnut-shaped laser beam. Our STEDYCON nanoscope (Abberior Instruments, Göttingen, Germany) is a user-friendly, powerful multi-colour confocal (488 nm, 561 nm and 640 nm) and STED (775 nm) system with a 2D STED resolution of <40 nm.
